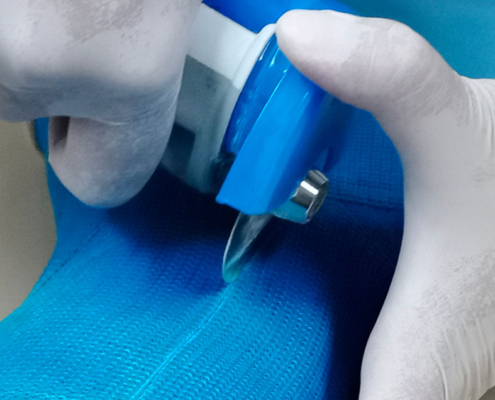
Penthrox® (Methoxyflurane) Monitoring
We provide monitoring of Penthrox® (methoxyflurane) in a range of departments including Emergency Departments to provide data to NHS Trusts and private hospitals to indicate that control measures in place are working effectively and staff are not at risk from the negative effects of over-exposure.
Methoxyflurane does not have a Workplace Exposure Limit1 (WEL), published by HSE. The only published value for an Occupational Exposure Limit for methoxyflurane is 15 ppm (8 h TWA), postulated by an Australian research group2, who consider this value to be a Maximum Exposure Limit. The value of 15 ppm (8 h TWA) is also referenced in a Materials Safety Data Sheet for methoxyflurane produced by Medical Developments International Limited3. In the absence of a published limit by HSE, the European Union or NIOSH, this value will be used for comparison purposes for the results presented in a report.
As the side effects of excessive exposure can include dizziness, memory loss, hepatic disorders and renal failure, it is vital that hospital staff work within safe workplace exposure limits.
How we monitor for Methoxyflurane exposure
We provide personal sampling tubes to be worn by staff throughout their shift. On completion of the monitoring program, sample tubes are returned to our lab for analyses.
A comprehensive report is subsequently provided for the trust. From the moment, you contact us a member of our account management team will be assigned to you and will guide you through the process from start to finish.
To find out more about our workplace exposure monitoring service for Penthrox® (Methoxyflurane) call us on 0333 015 4345 or email info@cairntechnology.com.
References
* https://medicaldev.com/wp-content/uploads/2023/08/SDS_0001_R9_SDS_Penthrox.pdf
** https://bnf.nice.org.uk/drugs/methoxyflurane/#prescribing-and-dispensing-information
 https://cairntechnology.com/wp-content/uploads/2025/06/cough-cropped.jpg
1080
831
Sharon Evans
https://cairntechnology.com/wp-content/uploads/2023/06/Cairn-logo_RGB-compact-3.jpg
Sharon Evans2025-11-10 14:20:442025-11-10 14:20:44Dust risks in hospitals
https://cairntechnology.com/wp-content/uploads/2025/06/cough-cropped.jpg
1080
831
Sharon Evans
https://cairntechnology.com/wp-content/uploads/2023/06/Cairn-logo_RGB-compact-3.jpg
Sharon Evans2025-11-10 14:20:442025-11-10 14:20:44Dust risks in hospitals