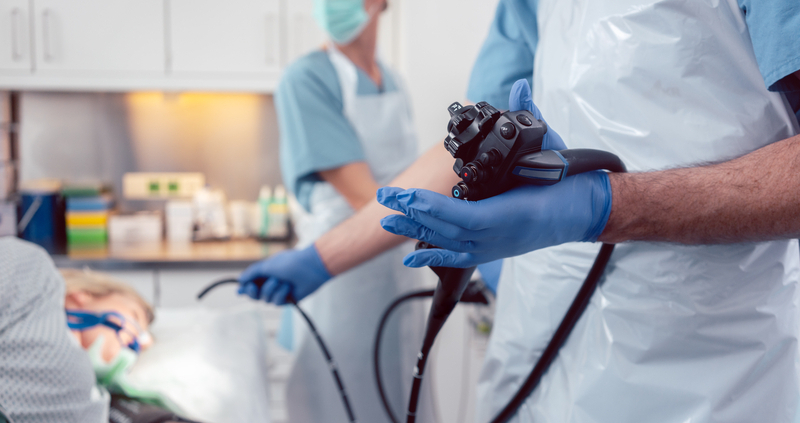
Decontamination of Endoscopes and Reusable Medical Devices
In this post, we’ll take a closer look at the specific decontamination processes that should be used for endoscopes and other reusable medical devices.
Levels of Decontamination
There are three levels of decontamination for medical devices: cleaning, sterilisation, and disinfection.
Every individual piece of medical equipment carries a different level of contamination risk – low, medium, or high. The level depends on how and where a device is used, and it also determines the appropriate decontamination procedure.
Read our full guide to the three levels of decontamination for infection control.
General Principles of Decontamination of Endoscopes
- Endoscopes come into contact with a patient’s intact mucous membranes, and they can sometimes breach gut mucosa. Endoscopes are also they’re likely to be contaminated with virulent or transmissible organisms. This means that endoscopes carry a medium to high infection risk.
- Because of the high infection risk, endoscopes will need to be cleaned and disinfected before use. Depending on their intended use, some endoscopes may need to go through an additional sterilisation process.
- Following decontamination, endoscopic equipment should be labelled and dated to confirm it’s clean and ready for use.
- Decontamination of endoscopes should only be carried out by staff who have been adequately trained in the correct procedures.
- To prevent cross-contamination, in the designated room for endoscopic decontamination, there should be a one-way flow between dirty returns and clean dispatch areas.
- There must be clear physical separation of clean and dirty procedures and areas, with specific handling, storing, and processing procedures for each area. Ideally, there’ll be two separate rooms – a dirty room, and a clean room.
Detergents and Disinfectants for Decontaminating Endoscopes
- To decontaminate endoscopes, use purpose-designed washers with single-use disinfectants. Start with a manual cleaning stage to ensure the endoscopes are visibly clean but note that manual disinfection processes are not suitable for endoscopes.
- Many best practice guidelines discourage the use of aldehyde- and alcohol-based disinfectants. Such disinfectants have fixative properties, which could bind certain proteins in the endoscope channels.
- Instead, only use detergents and disinfectants that are compatible with both the washer and the endoscope.
- Follow the detergent and disinfectant manufacturers’ instructions exactly, paying particular attention to the temperature and concentration recommendations.
Water Used for Decontamination
- Any water used in an endoscope washer disinfector should be free of particulate and chemical contamination, as well as any micro-organisms.
- If your local water supply delivers hard water, you may need to add in-line water softeners.
- Test the final rinse water at least once a week for microbiological levels.
Drying and Storing Endoscopes
- Decontaminated endoscopes should be stored separately from dirty endoscopes, ideally in a separate room.
- To reduce the risk of contamination, use dedicated endoscope drying and storage cabinets that are capable of delivering high-efficiency particulate filtered air (HEPA) at the correct temperature and flow rate.
- Never use alcohol solutions to assist in drying endoscopes, due to the fixative properties.
PPE for Staff Carrying out Endoscopy Procedures
- Any staff carrying out endoscopy procedures, along with all staff involved in endoscope decontamination, should wear the appropriate personal protective equipment (PPE).
- PPE procedures should advise on what PPE to use and where. They should also advise on the storage, application, and removal of equipment to reduce cross infection.
- You can read our full guide to PPE considerations.
Effective Decontamination For Infection Control in Endoscopy
The chemicals used in decontamination procedures can be harmful to human health. So all staff involved in endoscopy must comply with the Control of Substances Hazardous to Health (COSHH) Regulations wherever these chemicals are used.
Our workplace exposure monitoring services will help you identify the exposure risks for your endoscopy staff. Following the consultation, you’ll get a detailed report and actionable advice on the measures you can take to safeguard your staff’s health and wellbeing.
Learn more about our specialist workplace exposure monitoring services.