Keeping staff safe from inhalable and respirable dust
 If you manage a fracture clinic plaster room or orthotics laboratory, it is important to ensure that your staff are protected against the risks of inhalable and respirable dust.
If you manage a fracture clinic plaster room or orthotics laboratory, it is important to ensure that your staff are protected against the risks of inhalable and respirable dust.
This was underlined again only this month in an HSE eBulletin, which revealed that the Health and Safety Executive had recently fined a stone worktop manufacturer £60,000 and a wood supplier £40,000 for failing to protect workers from harmful dust exposure.
Plaster of Paris dust generated in plaster rooms and orthotics laboratories can lead to short-term and long-term respiratory problems.
So, it is vital that employers provide proper ventilation systems, appropriate respiratory protection, and carry out regular health surveillance to protect their workers.
Inhalable and respirable dust monitoring
We carry out workplace exposure monitoring in fracture clinic plaster rooms and orthotics laboratories to measure staff exposure to total inhalable and respirable dust.
Levels of these dusts need to be measured in accordance with the methods outlined in the HSE Publications MDHS 14/3.
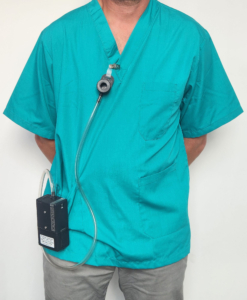 To ensure that this is done, we equip each member of staff with personal sampling pumps and monitor their exposure to dust during a normal working period.
To ensure that this is done, we equip each member of staff with personal sampling pumps and monitor their exposure to dust during a normal working period.
We then provide you with a report which details the staff exposure levels and outlines whether there are any causes for concern.
We recommend that this inhalable and respirable dust monitoring procedure should be carried out every 12 months to ensure continued compliance with Regulation 10 of the Control of Substances Hazardous to Health (COSHH) regulations 2002 (as amended).
To discuss our inhalable and respirable dust monitoring service or get a quote, please call us on 0333 015 4345 or email info@cairntechnology.com
What about staff exposure to Diisocyanates?
We can also help measure your staff’s exposure for Diisocyanates through our Biological Sampling service.
Diisocyanates are now used more frequently in fracture clinics because of the move away from Plaster of Paris towards synthetic products. They are also being used more widely in prosthetic laboratories where the use of resins is becoming more commonplace.
They are highly reactive substances which are potent respiratory and skin sensitizers and a common cause of asthma and allergic contact dermatitis.
The increase in Diisocyanate usage, along with the British Orthopaedic Association’s adoption of worker training guidance from the EU, has highlighted the importance of monitoring staff exposure.
We recommend that sampling should be carried out every 12 months to ensure continued compliance with Regulation 10 of the Control of Substances Hazardous to Health (COSHH) regulations 2002 (as amended).
If you are unsure whether or not your department is using products that contain Diisocyanates, our team can establish this on your behalf from the product material data sheets.
To discuss our Diisocyanate biological sampling service or get a quote, please call us on 0333 015 4345 or email us at info@cairntechnology.com
